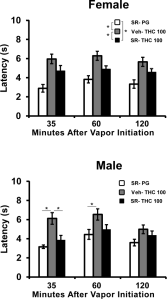
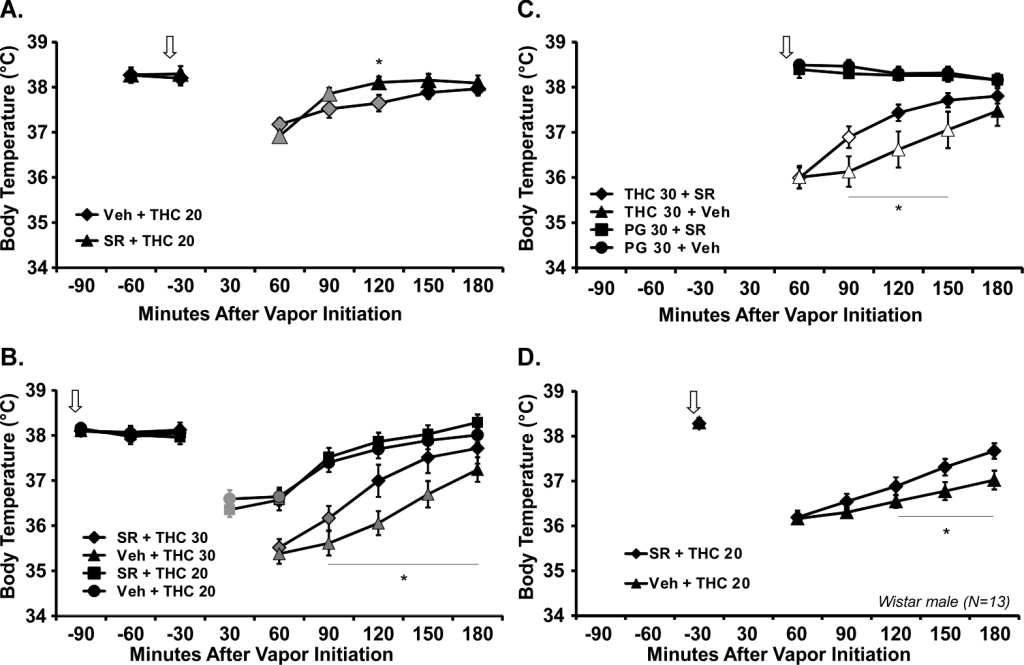
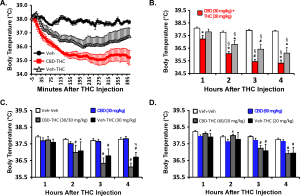
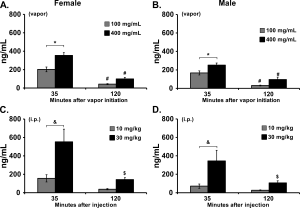
I recently published a Feature Article in eLife, with Nick Gilpin, on the NIH’s dismal response to the funding disparity which was identified in 2011. I also put in a small comment at Neuropsychopharmacology addressing the ACNP’s breast beating about diversity, equity and inclusion within the ranks of this highly selective academic society. It is not that I just noticed this issue all of a sudden. But I never really had the motivation to supply something formal in a traditional academic outlet.
Part of this is that it is a lot of work. The Correspondence to Neuropsychopharmacology, which ran only about a page, had to be crafted to make the essential points within the allowable limits for the publication type. It went to peer review and there was an editing and re-submission phase not unlike that for any manuscript. The eLife article started out as as massive beast of unruly text, which we eventually posted on psyArXiv. It took at least 6 months from when my co-author started kicking my butt into gear to write it until we were ready to post a pre-print and seek a publication venue. Sure, I’m a notorious procrastinator when it comes to getting manuscripts into final form for submission but part of this was the work involved. It’s all well and good to have ideas and the framework for a manuscript, but beating it into submittable shape takes effort. We selected a venue and a publication type that involved very heavy editorial input and requests for revisions. This took a lot more work.
All of this is work that could be spent on my “real” job, i.e., preparing scientific manuscripts for publication and writing yet more grant applications for NIH funding.
Others have been doing the same work in past years. My colleagues Carl Hart and Jean-Lud Cadet addressed the ACNP issue. Kafui Dzirasa has addressed these issues here and here. Stevens and colleagues published the very well-received Fund Black Scientists call. Valentine. Starbird. Guy and colleagues. There has been a growing number of publications from scientists in the past year or so.
These are not enough. It is my view that we need even more. Sure, the NIH Director’s statement on structural racism which was published March 1, 2021 is very welcome. But NIH is still punting this to committee work and further bureacratic responses. It is essential to keep the heat and our eyes on the NIH to redress the lack of equity in their research funding.
Every academic society and every academic journal should be publishing material on diversity, equity and inclusion within their subfields. Particularly all of the ones that receive funding from the NIH, but I am sure we will start seeing reports on the lack of equity across many funding bodies. Cancer Research UK seems to be admitting to a Ginther / Hoppe style problem, for example [PDF]. So who is going to do the work?
It’s up to us. Which returns me to the amount of work it takes to publish even the shortest commentary and certainly a lengthy review or feature article. We cannot expect people to engage in these activities as a sort of career charity. My point of the day is that we do not have to.
I have linked DEI publications in this post that all show up in a PubMed search. This is the first aspect of credit. If they show up in a PubMed search on your name, then they go into the total count that someone might be glancing at when giving you a look-see. (Note that this will not necessarily always be the case at every possible academic journal, depending on the type of publication format you are using.)
But there is another key factor which I am just verifying lately. These items go into the Web of Science database of citable content with their citations counted. This is huge. Yes, I know lots of you like Google Scholar but when you are dealing with the long established traditions and expectations of your Department and University, WoS is key. These DEI works will go into your publication counts. Any citations will go into your citation totals, including your h-index. I think this should be encouraging for anyone trying to decide whether writing a piece on the NIH’s efforts to respond to the inequities of grant award will be helpful or harmful to their career.