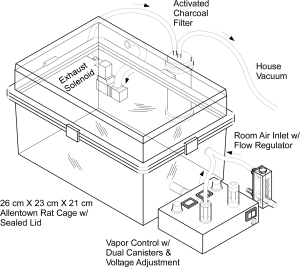
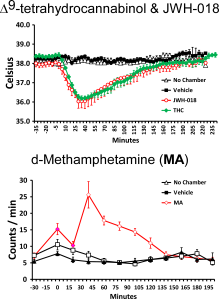
By this point the laboratory has published a number of papers showing that the vapor inhalation of Δ9-tetrahydrocannabinol (THC) reduces body temperature of rats. This was mostly done to validate our e-cigarette based method of drug delivery since hypothermia, along with anti-nociception, hypolocomotion and catalepsy, is a key marker of cannabinoid action in the rat (or mouse). This tetrad of signs was developed as a laboratory readout of THC-like effects before it was known where THC acted in the brain, before the endocannabinoid receptors were identified and before specific pharmacological tools were available to target the cannabinoid type 1 (CB1) receptor. Since we are a behavioral pharmacological lab one of the primary ways to validate is to show that effects depend on the dose of the drug that is administered, and we have done that using both time of exposure and concentration of the drug in the vapor to control dose. The pharmacological tools are available now so the secondary go-to validation for a behavioral pharmacological laboratory is to determine if antagonist drugs which block the ability of THC to interact with, e.g., the CB1 receptor, inhibit or block the effects of the inhaled drug.
A new paper (a pre-print version is available here) from the laboratory describes a several year journey of confusion about why a very simple experiment that “should” work, did not.
Nguyen, J.D., Creehan, K.M., Grant, Y., Vandewater, S.A., Kerr, T.M. and Taffe, M.A. Explication of CB1 receptor contributions to the hypothermic effects of Δ9-tetrahydrocannabinol (THC) when delivered by vapor inhalation or parenteral injection in rats. Drug Alcohol Depend, 2020, in press.
The compound SR141716 (it was approved as a treatment medication as “Rimonabant” but pulled from the market for suicidal ideation reasons) interacts with the CB1 receptor, both preventing THC and other agonists from working (i.e., as an antagonist) and potentially reducing constitutive activity of that receptor (called an “inverse agonist” effect). SR141716 works fine for the prevention of some THC-induced effects including the aforementioned tetrad of signs in rats. Most specifically including hypothermia. So long as the THC is injected. It also blocks anti-nociceptive effects of THC when injected OR when inhaled.
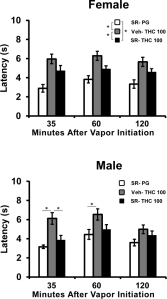
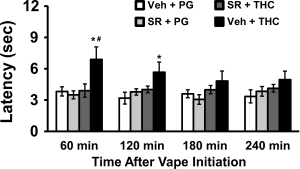
Figure 1 from this new paper shows the speed with which the rat tail is withdrawn from a 52 degree C water bath after a 30 minute inhalation session. When the vapor is from the PG vehicle, the tail is withdrawn within about 3 sec (open bar). After THC (grey bars) inhalation this slows to over 6 seconds in male and female rats. This is the anti-nociceptive effect of THC when delivered by inhalation but the magnitude is similar to what is produced by THC injection. Pretreatment with SR141716 (black bars) before the inhalation session entirely prevents the anti-nociceptive effect of THC in male rats entirely and greatly reduces it in female rats. So far, so good, and actually we showed something similar in our very first paper, Nguyen et al 2016 (see Figure 4B).
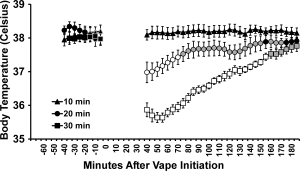
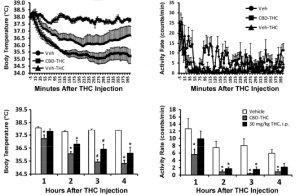
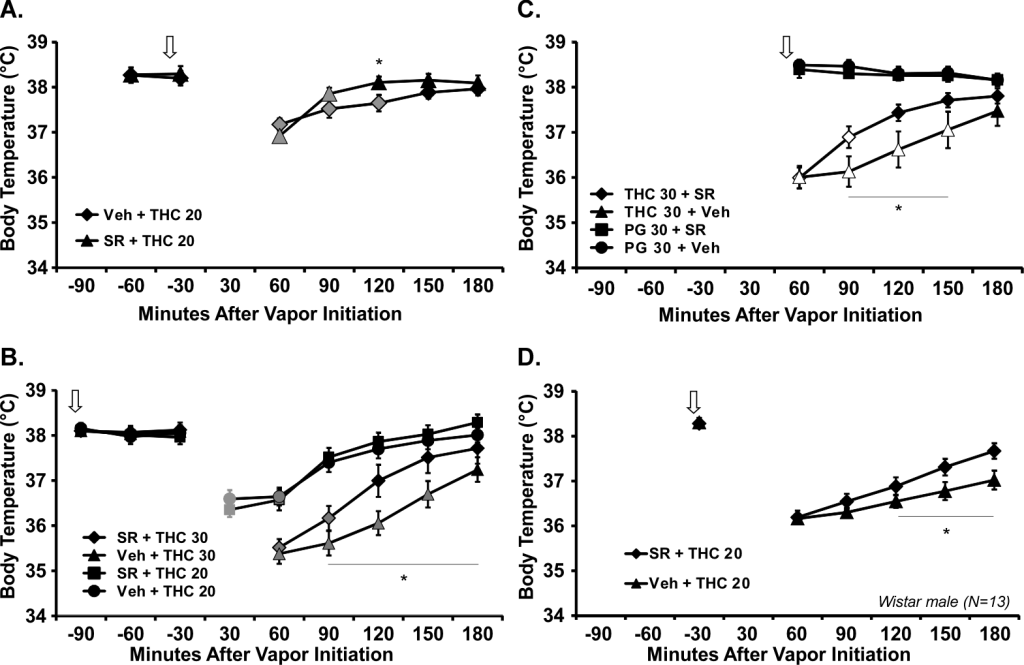
Dating back to our very original studies, this is not what happens with the temperature response. Figure 2 from the new paper shows (Panels A, B, D) that SR141716 administered prior to THC inhalation does not affect the initial drop in body temperature, observed immediately after the 20-30 minute session.
What SR does do is slightly accelerate the return of body temperature to the normal range. To walk through the logic of these first panels, the study in A suggested perhaps the SR simply wasn’t effective until 90 minutes later so in B we moved the pre-treatment interval to 90 minutes before inhalation. No difference. There’s always a concern about dose so in Panel D we increased from 4 mg/kg SR141716 to a 10 mg/kg dose, and had no change. In Panel C, we injected the SR after the vapor session and again, no change in the overall picture, suggesting that even with pre-vapor administration of the SR141716, essentially the same level of activity was present in the post-vapor monitoring period.
The rest of the paper describes more experiments along the same theme- trying to give the antagonist the best chance to “work” and further confirming that it does work to prevent body temperature changes…..just so long as the THC is injected (either intraperitoneally or intravenously).
None of the usual pharmacological explanations related to antagonist/agonist dose, speed of uptake into the brain or competition for binding sites seem to be at work here. We’ve altered dose and injection route. We’ve evaluated both sexes and two different rat strains. Studies range across animal age (and therefore size) and their treatment history, including a chronic THC dosing group that exhibited partial tolerance to the THC inhalation. We have conducted vehicle inhalation controls, which do not alter body temperature, and thus there cannot be any role of the vapor exposure by itself. We used an alternate antagonist/inverse agonst drug and produced similar qualitative results.
Most convincingly, of course, the effects of the antagonist differ between the temperature and the nociceptive effects of THC inhalation.
Scientifically this is a nagging mystery. We’ve thrown effort at these studies over several years now, and have tried other manipulations that fail to resolve the question to our satisfaction. The literature on injected THC is reasonably robust but there are few investigations reported for inhaled exposure. What exists is limited by the experimental design. For example, Wilson and colleagues (2002) showed that the temperature response to inhaled THC could be attenuated by SR141716 pre-treatment in mice, but they only measured temperature at a single time-point after inhalation, with locomotor, nociception and catalepsy tests being performed first. It cannot be determined, therefore, if the temperature response at some earlier timepoint was unaffected by the SR141716 pre-treatment. This partial effect also conflicts with a complete blockade of the temperature response to inhaled THC in a different strain of mice reported by Marshell and colleagues (2014).
Sadly, this is the sort of mystery that doesn’t really justify a lot more effort and money to resolve. While important and useful for validation purposes, the hypothermic response to THC does not appear to have much translational value. It is not clear that human body temperature is affected by THC ingestion at all, nor what health implications this might have even if humans do experience temperature reductions.
This paper is one that I feared might not ever be publishable. It presents a bit of a mystery and does not actually solve it. This is not typically what is found to be publishable in academic science reporting. But from a conduct of science perspective it is really important to get out there. Just as we followed this frustrating path after starting from a expectation of rapid pharmacological validation of our method, others might likewise wish to validate their inhalation models. E-cigarette use for delivering cannabinoids continues to be very popular with both medical and recreational consumers of cannabis (via extracts). This encourages researchers to try to adopt similar methods to explore any possible implications for health or well being. There are other labs doing similar work already and they are, in many cases, rooted in behavioral pharmacological thinking as much as we are. At the very least, our paper serves as a warning that things may not be simple.
More usefully, perhaps someone will come up with a brilliant insight about where our thinking has led us astray. We could be missing something incredibly simple that explains all of this in a satisfying way. That is the absolute brilliance of the grand enterprise of science, i.e., that publishing work leads to someone else either confirming, contradicting or explaining our current state of knowledge.